Accessing Allergan: A map for Selling to Allergan
February 24, 2020 | Xhermi Trimi |
Industry Articles
Note: This article was originally written for 2019 publication, and has since been updated.
Allergan is a multinational company, based out of Ireland. The company develops, manufactures, and distributes Allergan-branded medical devices, biologics, surgical products and pharmaceutical products in over 100 countries. Their flagship product is Botox (botulinum toxin) and they ranked 20th on Pharm Exec’s 2019 list of top pharmaceutical companies (based on 2018 product sales) [1].
Allergan, as we know it today, is the result of a series of mergers, acquisitions, and name changes. The company was initially founded under the name Watson Pharmaceuticals in 1983 in Libertyville, Illinois [2]. In 2012, they became Actavis plc after acquiring that company. One year later, they acquired an Irish Registered company (Warner Chilcott) in a transaction structures as a tax inversion to Ireland [3]. In 2015, this Irish-registered Activis plc acquired the US-registered companies (including Allergan Inc and Forest Labs) and renamed itself Allergan [4]. Recently, in 2019, the US company AbbVie acquired Allergan for $63B USD [5]. Prior to that, in 2016, Pfizer had proposed a $160B merger will the company, which was blocked by the US Treasury due to the fact that the transaction was structured as a tax inversion [3].
Business Overview
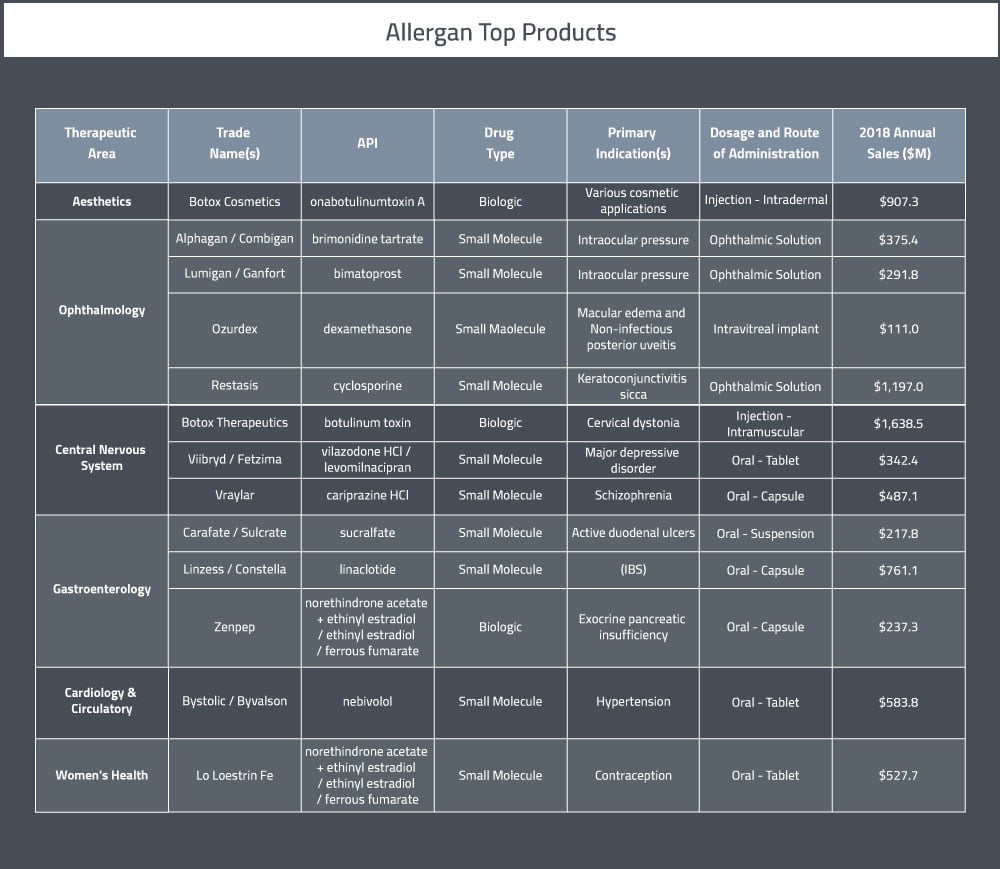
Allergan has operations in over 100 countries. At the end of 2018, Allergan had approximately 16,900 employees—approximately 2,150 were in R&D functions; 4,900 has functions supporting Cost of Goods Sold functions; 8,450 supported sales, marketing and distribution functions; and 1,400 supported administrative functions [4]. Their four key therapeutic areas are medical aesthetics, eye care, central nervous system and gastroenterology. Allergen’s main products (each with US sales in excess of $200M) are listed in Table 1 below.
In 2018, Allergan’s pharmaceutical sales totalled about $14.7B and their R&D expenditures in that year were $1.6B [1]. Allergan’s R&D effects are split across four main areas of focus [6]:
- the application of proprietary drug-delivery technology for new product development in specialty areas;
- the acquisition of mid-to-late development-stage brand drugs;
- early stage collaboration arrangements;
- and the development of sustained-release, semi-solid, liquid, oral trans-mucosal, transdermal, gel, injectable, and other drug delivery technologies and the application of these technologies to proprietary drug forms.
There are currently 134 active clinical trials being sponsored by Allergan. They currently have 8 products in Phase III (expected launch years ranging from 2020-2023) and 5 products in Phase II (expected launch years ranging from 2023-2025).
Outsourcing History
Manufacturing
Allergan manufactures some of their finished products, raw materials, active pharmaceutical ingredients, and intermediate ingredients at their own plants. However, they are also dependent on third parties for the supply of many of their finished products as well as for the supply of raw materials (both API and inactive pharmaceutical ingredients) necessary to develop and manufacture those products. A significant portion of those raw materials are provided by foreign suppliers.
Examples of past manufacturing partners of Allergan are Teva for Lo Loestrin and Patheon for Viberzi [6]. Both of these partners utilize single manufacturing facilities for the products they produce.
Clinical / R&D
Allergan has a long history of acquiring promising clinical-stage companies—these acquisitions are frequently recorded as part of their annual R&D expenses. For example, in 2018 they acquired Bonti Inc, who are developing and commercializing novel, fast-acting neurotoxins for aesthetic and therapeutic applications [6]. Also in 2018, Allergan acquired Elastagen Pty Ltd, a company developing several medical and cosmetic treatments including a novel filler [6]. In 2016, Allergan acquired both Vitae Pharmaceuticals an ForSight VISION5 Inc [6]. Vitae has several promising dermatology treatments (for psoriasis and atopic dermatitis, for example) and ForSight VISION5 has candidates for eye care (e.g. a peri-ocular ring for extended drug delivery and reducing elevated intraocular pressure (“IOP”) in glaucoma patients).
Commercialization
Allergan has a history of entering into agreements with third parties for the development, and commercialization of their proprietary compounds in specific markets. For example, in April 2015 they entered into an exclusive agreement with Dong-A ST to develop and market their experimental fatty liver disease product (cenivriviroc) in Korea [7]. Similarly, they will often enter into agreements for the development, commercialization or licensing of experimental drugs or drug delivery technologies owned by clinical-stage companies. For example, in October 2016, Allergan licenced Urogen Pharma’s RTGelTM delivery system technology for use with neurotoxins [8]. In that contract, they made an upfront payment of $17.5 million, and agreed to development and commercial milestones as well as royalties on net sales of any finished products.
Allergan frequently acquires clinical-stage companies with promising compounds or technologies. In 2016 they made several acquisitions to expand their gastrointestinal disease portfolio. Specifically, they acquired Tobira Therapeutics for up to $1.695 billion and Akarna Therapeutics for $50 million, thereby adding three nonalcoholic steatohepatitis (NASH) drug candidates to its GI R&D pipeline [9]. Later in the same year, they also acquired Motus Therapeutics for $200 million upfront (plus undisclosed sales milestone payments). Similarly, also in 2016, they acquired LifeCell, a regenerative medicine company, for $2.9 billion in order to enter into the field of regenerative medicine [10].
Have a Zymewire account? Click here to view Allergan's global activity!
Drug Development Sites
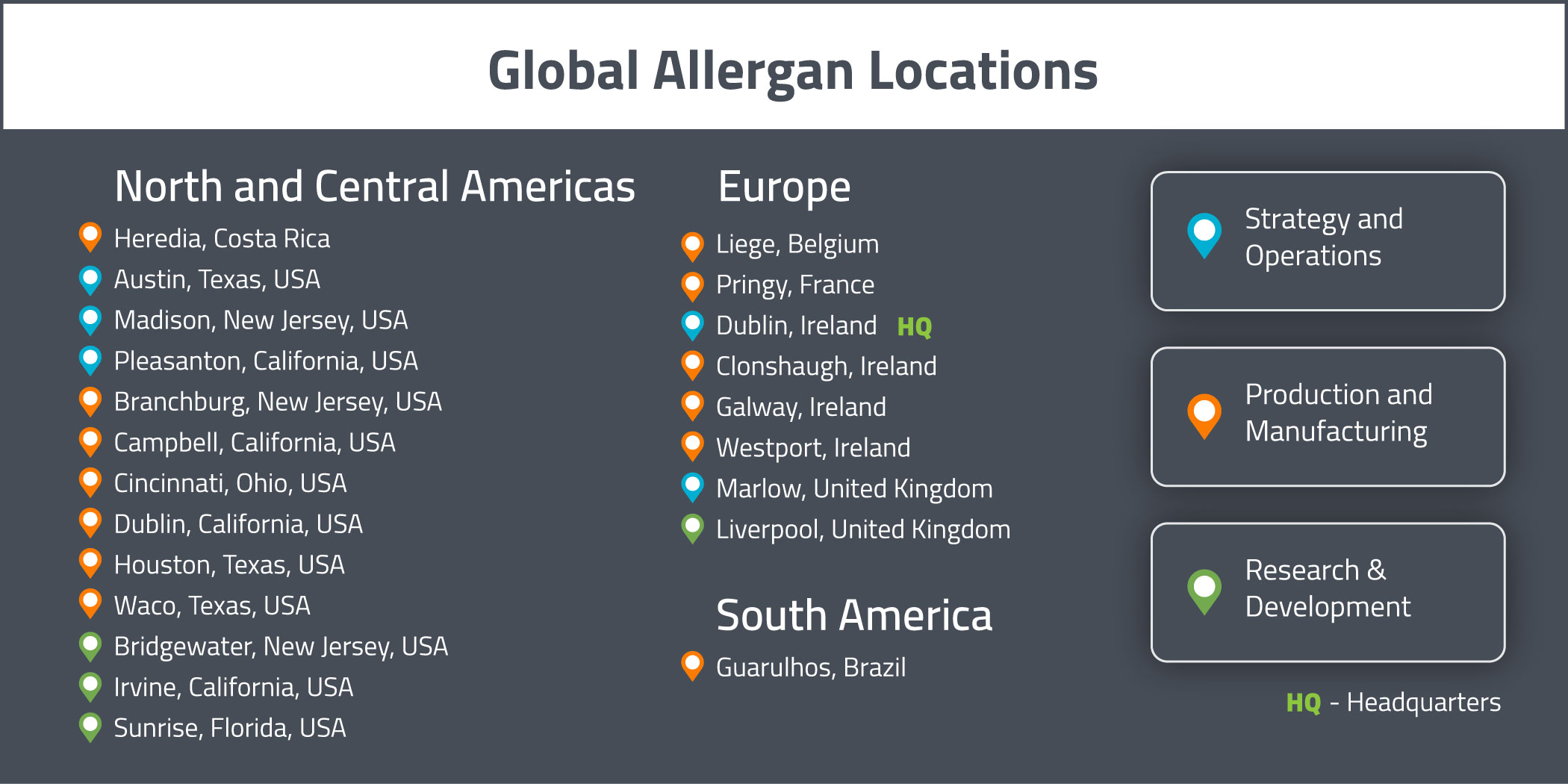
Allergan conducts their operations at a number of owned and leased properties internationally. The functions of these properties include R&D, manufacturing, distribution (including warehouse and storage), sales & marketing and administration. A summary of their major properties can be found below. Their global headquarters is located in Dublin, Ireland.
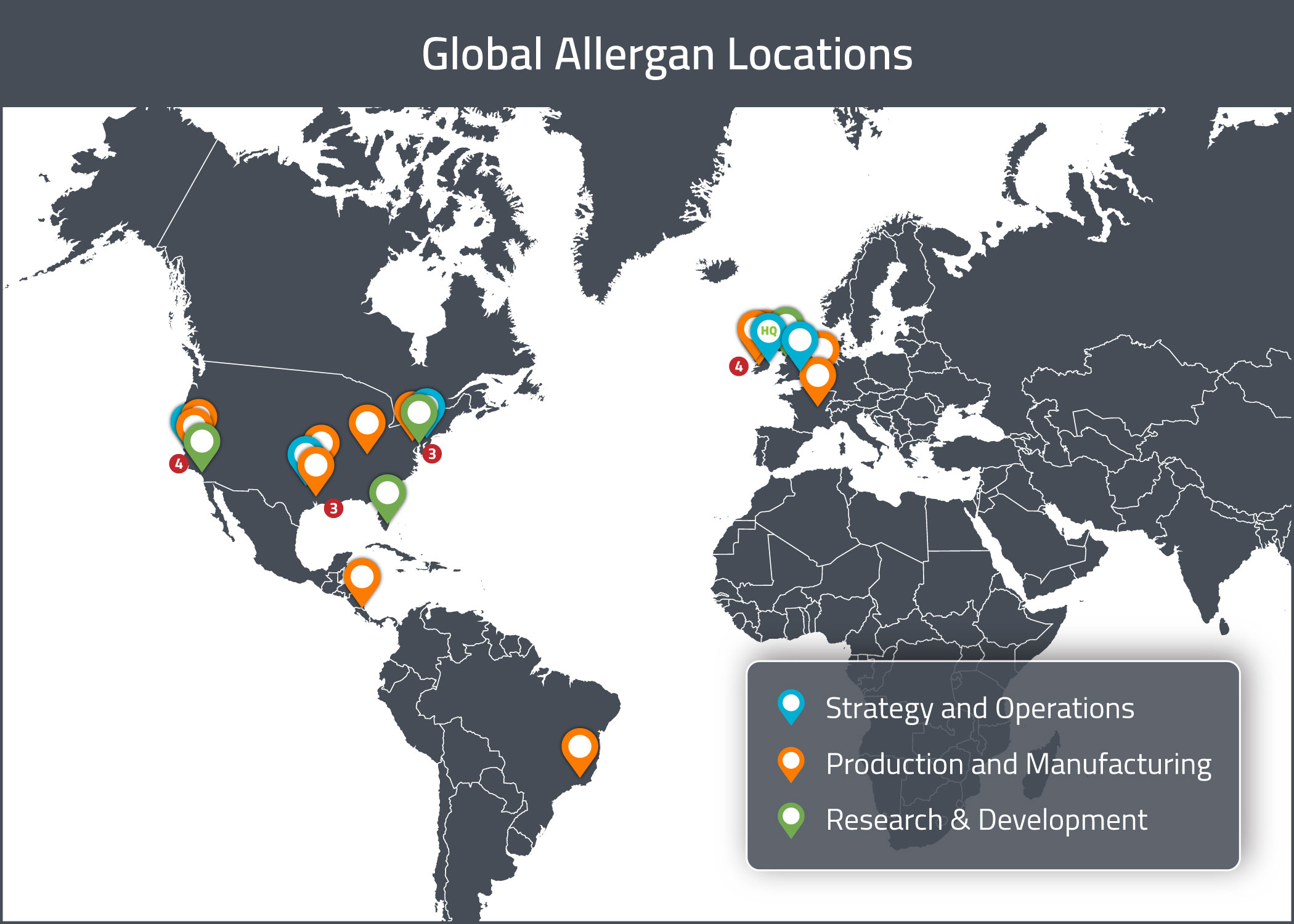
Research and Development
Allergan’s research and development efforts are based out of sites in:
- Bridgewater (New Jersey, USA)
- Irvine (California, USA)
- Liverpool (United Kingdom)
- Sunrise (Florida, USA)
Their R&D site in Speke, Liverpool, is a Center of Excellence for Biologics development and hosts a team of over 140 researchers.
Manufacturing
Their major manufacturing sites are located in:
- Branchburg (New Jersey, USA)
- Campbell (California, USA)
- Cincinnati (Ohio, USA)
- Clonshaugh (Ireland)
- Dublin (California, USA)
- Galway (Ireland)
- Guarulhos (Brazil)
- Heredia (Costa Rica)
- Houston (Texas, USA)
- Liege (Belgium)
- Pringy (France)
- Waco (Texas, USA)
- Westport (Ireland)
Administration
Allergan’s main administrative sites are located in:
- Austin (Texas, USA)
- Madison (New Jersey, USA)
- Marlow (United Kingdom)
- Pleasanton (California, USA)
Decision Making
Decision making has been classified into three sections: clinical outsourcing, manufacturing and logistics. This division should facilitate outreach efforts by different types of companies looking to conduct business with Allergan.
The Americas
In general, decision making for the Americas is based out of the United States, in Madison (New Jersey), Irvine (California), Austin (Texas) and Washington (DC). Allergan’s US Administrative Headquarters are located in Madison (New Jersey). Their “Customer Operations Center of Excellence” is located in Austin (Texas) and their US Government Affairs Office is located in Washington (D.C.). As a result, several of their American decision makers are based out of these locations. They also have an important site for decision making in Irvine (California).
Clinical Outsourcing
Madison (New Jersey) is where you’ll find multiple Directors of Clinical Operations, a Director of Clinical Vendor Oversight and a Director of Global Clinical Trial Management. In Irvine (California), you’ll find several more Directors of Clinical Development, multiple Directors of Clinical Operations, a Director of Global Clinical Trial Management, multiple Vice Presidents of Global Clinical Trial Management and a Director of Vendor Management & Oversight.
Manufacturing
In Madison (New Jersey), there is a Director of Third Party Manufacturing and a Director of Clinical Manufacturing. In Irvine (California), there are multiple Manufacturing Managers and there is a Director of Manufacturing. Finally, in Waco (Texas), there is a Director of Manufacturing and multiple Manufacturing Managers.
Logistics Operations
The Madison (New Jersey) site is where you’ll find several logistics decision makers including the Director of North American Logistics as well as multiple Directors of Global Clinical Supply Management, Directors of R&D Clinical Procurement, Procurement Managers and Supply Chain Managers. In Irvine (California), there is a Vice President of Global Sourcing & Procurement, a Director of Global Procurement and multiple Procurement Managers. Elsewhere, in Canada (Toronto), there is a Director of Supply Chain.
Have a Zymewire account? Click here to view Allergan's global activity!
Europe
For Europe, the majority of Allergan’s key decision makers are mostly based out of the United Kingdom. Allergan’s UK headquarters is located in Marlow, Buckinghamshire. This headquarters is the base for Allergan’s European, African and Middle Easter Regulatory Affairs functions, as well as several of their international commercial and support functions and their global clinical development teams.
Clinical Outsourcing
The majority of clinical decision making for Europe appears to take place in the United Kingdom. In Marlow (UK), there are multiple Clinical Trial Managers and multiple Directors of Clinical Development. In London, there are several Directors of Clinical Development, as well.
Manufacturing
Allergan’s manufacturing decisions are based out of Ireland, in the United Kingdom. Allergan has three manufacturing sites in Ireland: Clonshaugh, Galway and Westport. Decision making seems to take place primarily out of Westport, as that is where you will find multiple Directors of Manufacturing and multiple Manufacturing Managers.
Logistics Operations
Once again, for logistics operations, there are many decision makers based out of the United Kingdom (specifically, Ireland). In Dublin (Ireland), there is a Director of Global Logistics, multiple Directors of Global Procurement and multiple Directors of Supply Chain. In Westport (Ireland), there is a Director of Global Clinical Supply and in Dundalk (Ireland), there are multiple Procurement Managers. There are also some decision makers elsewhere in Europe, such as a Logistics Manager for Nordics in Stockholm (Sweden).
APAC
For APAC, Allergan’s decision makers are spread out across several countries. There are decision makers in Singapore, China, India, Japan and Australia. There does not appear to be a central location for APAC decision making.
Clinical Outsourcing
With regards to clinical outsourcing, you’ll find some decision makers in Beijing (China). Specifically, Beijing is where you’ll find the Head of China Clinical Development and multiple Clinical Managers. There are also multiple Clinical Mangers in Mumbai, India. Elsewhere, in Singapore, you’ll find multiple Directors of Clinical Operations.
Manufacturing
Manufacturing decisions for APAC are probably handled mostly out of the UK headquarters. However, there are a few decision makers scattered across APAC. For example, you’ll find a Director of Manufacturing in the Bengaluru Area of India
Logistics Operations
In India, there’s a Director of Supply Chain and a Supply Chain Manager in the Bengaluru Area. In Australia, there is a Supply Chain Director for Asia Pacific in Sydney. Finally, in Japan, there is a Head of Supply Chain in Tokyo.
Have a Zymewire account? Click here to view Allergan's global activity!
Innovation
Internationally, Allergan fosters innovation through its “Open Science” strategy [11]. This strategy involves strategically investing in innovation to increase R&D efficiency in unexpected places such as, biotech, specialty pharma and academia or under-served disease areas. Along the same vein, Allergan is also fostering innovation internationally through grants such as the DCU Allergan Innovation Award. The 2018/19 winner of the grant (from Dublin, Ireland) was awarded €5,000 to advance this research involving stem cells glycosylation and its impact on heart disease [12].
In the United States, Allergan has recently joined the in Cambridge, Massachusetts life sciences R&D hub. Their facility in Kendall Square opened its doors in 2019 [13]. Elsewhere in the world, in High-Tech Zone of Chengdu (China), Allergan is fostering innovation in a different way. In early 2019, they opened a state-of-the-art medical aesthetics Innovation Center for training practionners (their goal is to train 3,000 professionals per year) and providing education and experience centers for consumers [14].
Where can I find more information like this?
If you would like a simple solution for keeping an eye on drug sponsor companies, such as Allergan, without relying on a database and generic lists of leads each week, we at Zymewire are here to help. Reach out today, and stay tuned for the next instalment of Sponsor Atlas: Selling to the Pharma Giants. If you enjoy these articles, please feel free to give them a share!
To view Part 8 of the Sponsor Atlas series, All About AbbVie: A map for Selling to AbbVie, click here!





.png?width=500&name=Q3%202024%20Biopharma%20Recap%20(Zymewire).png)

Comments